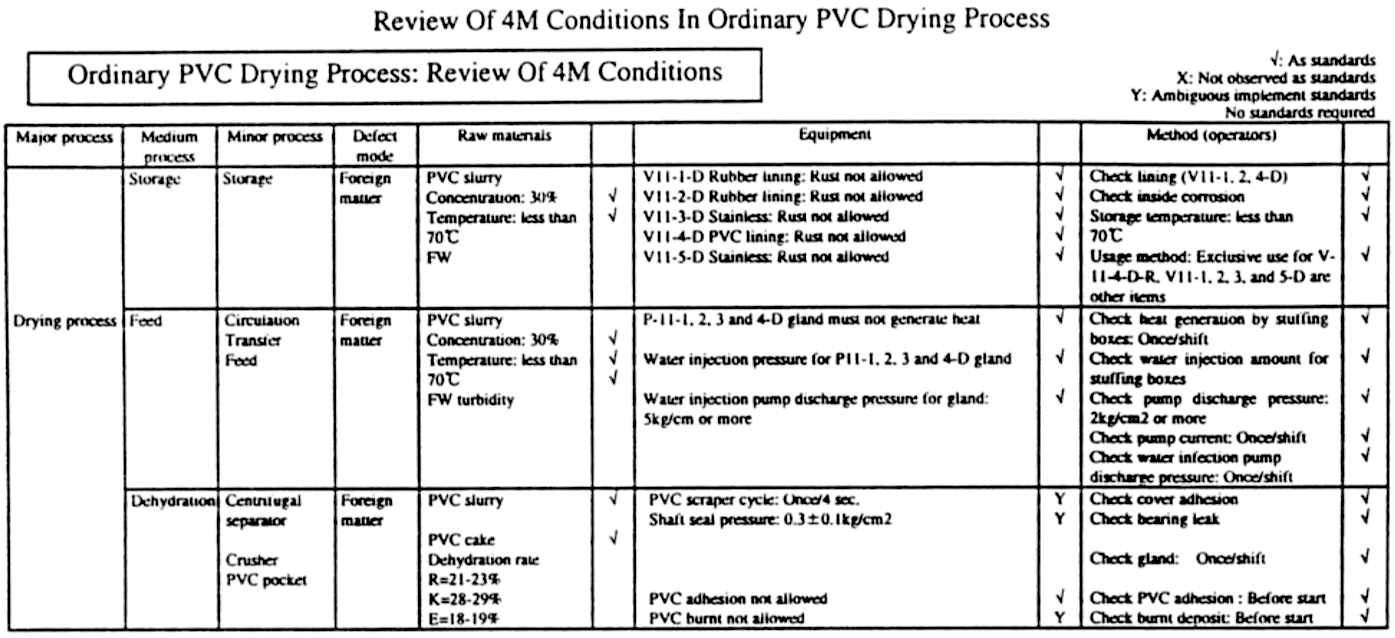
In this step 6, we actually implement the improvements proposed in Step 5. The results are then assessed at prescribed intervals to ensure that they satisfy the product’s designed quality characteristics (see Figure “Eliminating Flaws in 4-M Conditions through Kaizen”).
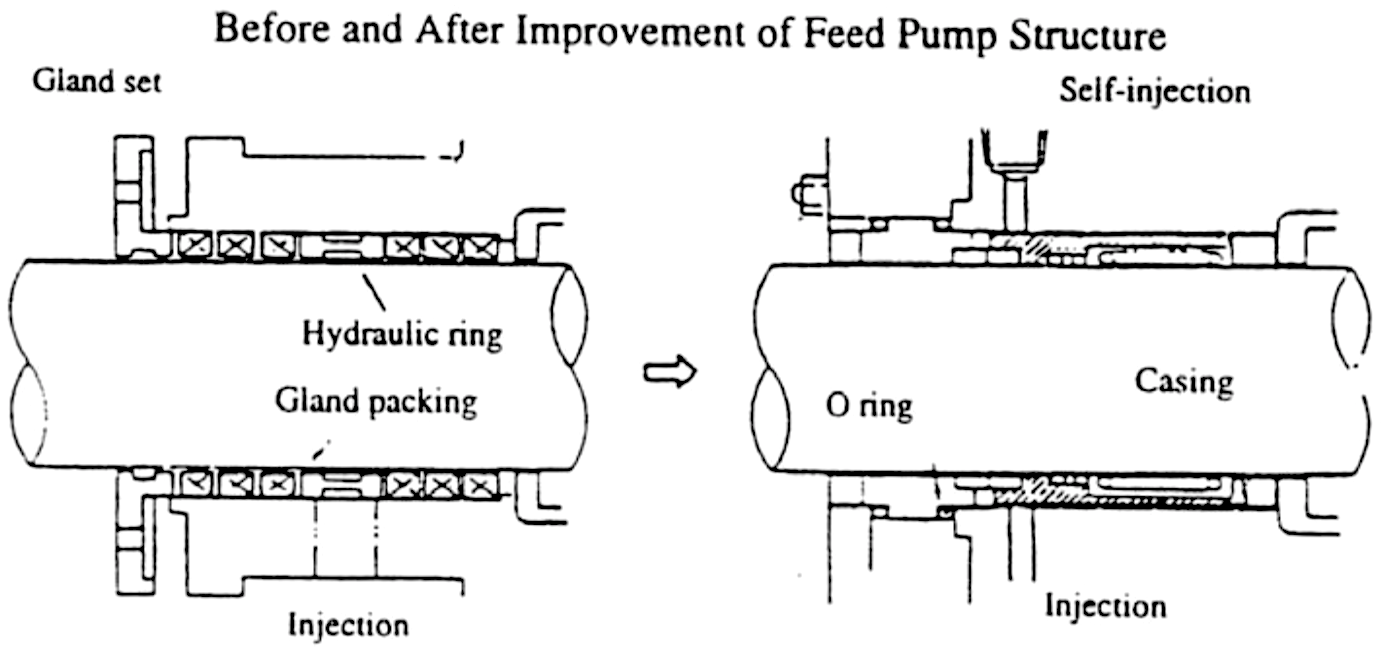
On completing Step 6, we revise the conditions and standards identified in Step 3 (Investigate and analyze 4-M conditions) in order to establish zero-defect 4-M conditions (see Figure “Finalizing 4-M Conditions”).